Describe Using a Diagram How a Continuous Spectrum Is Formed
Different types of atoms have different energy levels. Since electrons are much lighter than atoms irregular thermal motion.
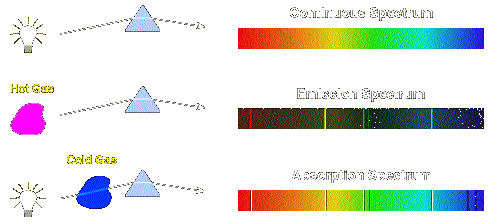
Continuous Spectrum Vs Line Spectrum Continuous Spectrum
A continuous spectrum is produced when all the colors of a rainbow from red to violet are present.
. The term continuous spectrum is mostly found in physics and mainly involves light and colors found therein. Which radiation has a wavelength greater than visible light in this spectrum. Block Diagram of X-Ray OperationWorking of X-Ray Machine High voltage source and high voltage transformer.
Perfectly white light shined through a prism causes dispersion of the light and we see a. Essentially light bends refracts when passed through a prism which is why we can see the rainbow after it as rained. As we can see the spectrum analyzer is composed of components like RF attenuator mixer IF filter detector sweep generator local oscillator and display unit.
Which color has the highest frequency in the visible part of the solar spectrum. Electrons in an atom occupy the ground state. Such spectra are emitted by any warm substance.
Comes form dense gases or solid objects that radiate heat away through light production. Learn the sources of white light. In physics a continuous spectrum usually means a set of attainable values for some physical quantity such as energy or wavelength that is best described as an interval of real numbers as opposed to a discrete spectrum a set of attainable values that is discrete in the mathematical sense where there is a positive gap between each value and the next one.
This is the only series of the line in the electromagnetic spectrum that lies in the visible region. A continuous spectrum contains many different colors or wavelengths with no gaps. Describe how an absorption spectrum is formed.
Spectrum seems smooth and continuous because light is emitted over a broad range on wavelengths. These frequencies of photon of light are then missing in the spectrum produced instead there are dark lines showing the. There are two main types of spectra.
Write one merit and demerit of infrared and ultraviolet radiations. The radiation frequency is key parameter of all photons because it determines the energy of a. X-rays also known as X-radiation refers to electromagnetic radiation no rest mass no charge of high energies.
An emission line is formed when the electron falls back to a lower energy state releasing a photon. When light passes through gas in the atmosphere some of the light at particular wavelengths is. Continuous spectra of electromagnetic radiation.
We can use Bohrs model of the atom to understand how spectral lines are formed. An absorption spectrum is formed when a blackbody source which produces a _____ spectrum is viewed through a _____ gas. Continuous Spectrum Emission Spectrum and Absorption Spectrum.
The concept of energy levels for the electron orbits in an atom leads naturally to an explanation of why atoms absorb or emit only specific energies or wavelengths of light. Geometrically the output signalytis a reflection of the input signalxt about the vertical linet0. It consists of unbroken luminous bands of all wavelengths containing all the colours from violet to red.
ν 109677 1 2 2 1 n 2 This series of the hydrogen emission spectrum is known as the Balmer series. The higher the temperature the more rapid the motion. To illustrate the effects of time reversal an example is provided in Figure 21.
The term R H is called the Rydberg constant for hydrogen. An emission spectrum is formed when a _____ gas is viewed directly. Block Diagram of X-Rays machine.
Emission lines refer to the fact that glowing hot gas emits lines of light whereas absorption lines refer to the tendency of cool atmospheric gas to absorb the same lines of light. These spectra depend only on the temperature of the source and is independent of the characteristic of the source. The diagram on the next page demonstrates absorption and emission of photons by an atom using the Neils Bohr model of a hydrogen atom where the varying energy levels of the electron are represented as varying orbits around the nucleus.
Spectra also help us understand how atoms absorb different light energies to provide the color we see. This formula is given as. Incandescent solids liquids Carbon arc electric filament lamps etc give continuous spectra.
So let us now understand the operation performed by each block individually. Lets look at the hydrogen atom from the perspective of the Bohr. Continuous and line spectra and while these are generally different it is possible to have both.
X-Ray Spectrum Characteristic and Continuous. The figure below shows the block diagram representation of a spectrum analyzer with digital display. High voltage source is responsible for providing high voltage to the HV transformer for a decided time.
Use the colored. Heat is the irregular motion of electrons atoms and molecules. 21 In other words the output signalyt is formed by replacingtwith tin the input signalxt.
Terms in this set 13 Three Different Types of Spectra. Other articles where continuous spectrum is discussed. Analyse the diagram of solar spectrum and write answer.
When white light is shone through a gaseous form of an element the electrons absorb photons of light of specific energy. White light is a mixture of all wavelengths of the visible spectrum occurring both naturally and artificially. This is not a continuous spectrum as only light of specific frequencies and specific colours are produced.
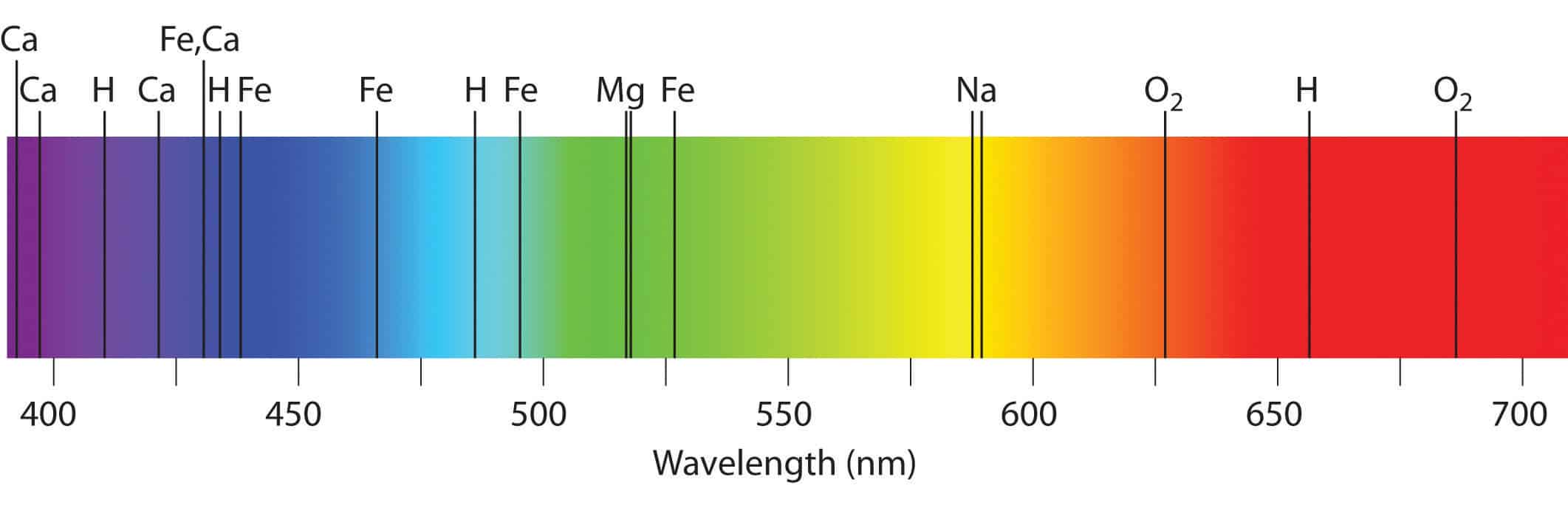
Hydrogen absorption and emission lines in the visible spectrum. A continuous spectrum results when the gas pressures are higher so that lines are broadened by collisions between the atoms until they are smeared into a continuum. As shown in this diagram.
We may view a continuum spectrum as an emission spectrum in which the lines overlap with each other and can no longer be distinguished as individual emission lines. The HV transformer produces 20 KV to 200 KV at the OP. Assume an emission spectrum and draw arrows indicating the possible transitions.
X-rays are high-energy photons with short wavelengths and thus very high frequency.

Formation Of Spectral Lines Astronomy

No comments for "Describe Using a Diagram How a Continuous Spectrum Is Formed"
Post a Comment